
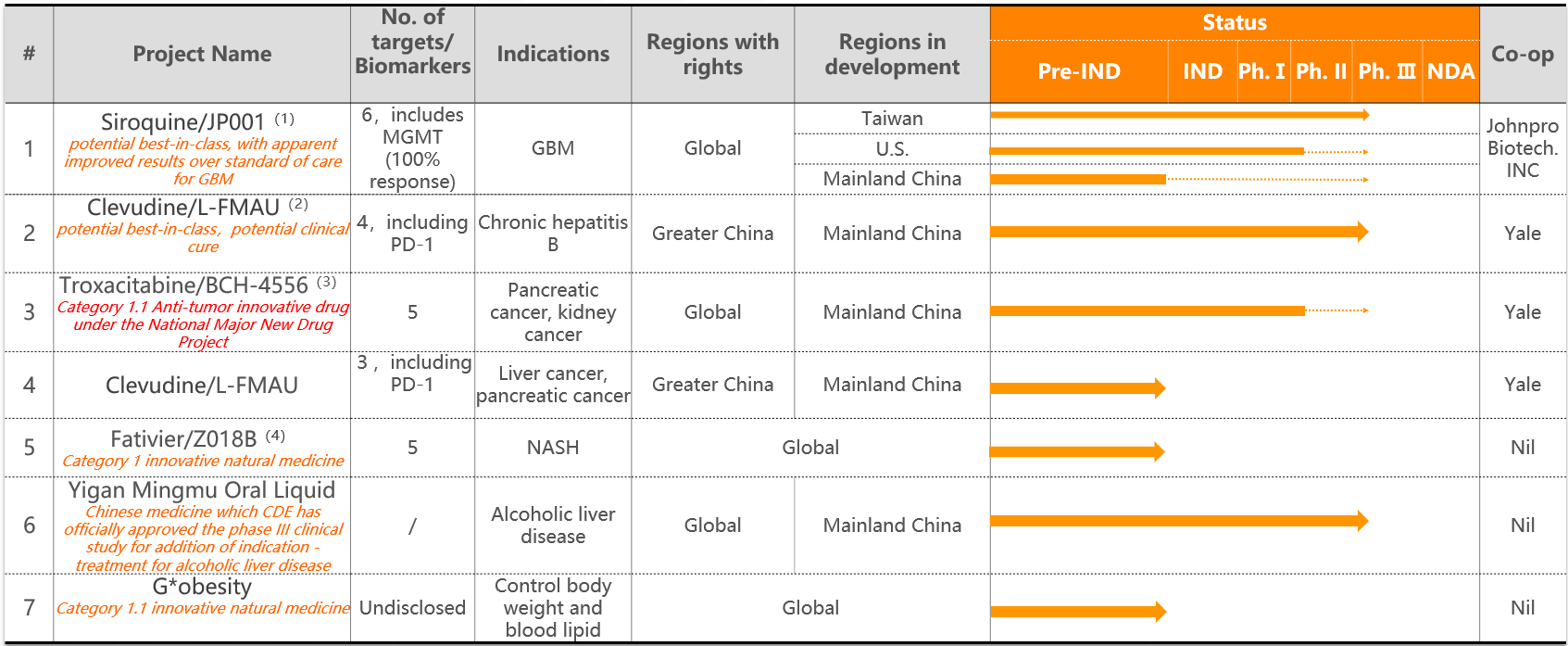
Notes:
(1)An international multi-center, open-label Phase II/III clinical study is being carried out, and it is planned to be implemented in Taiwan, the United States and mainland China. Other solid tumor indications such as drug-resistant renal cancer, sarcoma cancer, bladder cancer, and liver cancer will be developed in the future.
(2) In animal model tests of pancreatic cancer and liver cancer, clevudine has shown obvious anti-tumor effects, and has synergistic effects with a variety of anti-tumor drugs already on the market and the company's new drugs troxacitabine and JP001. Patients with liver cancer caused by hepatitis B have better results. Preliminary studies have found multiple targets and biomarkers.
(3) Phase I-III trial approvals have been obtained; Phase I solid tumor research has been completed, and mechanism-related research has been supplemented, and new biomarkers/targets have been discovered; renal cancer single drug (including non-clear cell carcinoma) and pancreatic cancer combination are being developed. with Phase II-III studies.
(4) The IND application stage.





